Gas laws
The early gas laws were developed at the end of the 18th century,
when scientists began to realize that relationships between the
pressure, volume and temperature of a sample of gas could be obtained
which would hold for all gases. Gases behave in a similar way over a
wide variety of conditions because to a good approximation they all have
molecules which are widely spaced, and nowadays the equation of state
for an ideal gas is derived from kinetic theory.
The earlier gas laws are now considered as special cases of the ideal
gas equation, with one or more of the variables held constant.
Boyle's law
Boyle's law shows that, at constant temperature, the product of an ideal gas's pressure and volume is always constant. It was published in 1662. It can be determined experimentally using a pressure gauge and a variable volume container. It can also be found through the use of logic; if a container, with a fixed number of molecules inside, is reduced in volume, more molecules will hit the sides of the container per unit time, causing a greater pressure.As a mathematical equation, Boyle's law is:
This is known as Boyle's law which states: the volume of a given mass of gas is inversely proportional to its pressure, if the temperature remains constant. Mathematically this is:

where k is a constant (NOT Boltzmann's constant or Coulomb’s constant).
Charles' law
Gay-Lussac's law
Gay-Lussac's law, or the pressure law, was found by Joseph Louis Gay-Lussac in 1809. It states that the pressure exerted on the sides of a container by an ideal gas of fixed volume is proportional to its temperature.Avogadro's law
Avogadro's law states that the volume occupied by an ideal gas is proportional to the number of moles (or molecules) present in the container. This gives rise to the molar volume of a gas, which at STP is 22.4 dm3 (or litres). The relation is given byCombined and ideal gas laws
The combined gas law or general gas equation is formed by the combination of the three laws, and shows the relationship between the pressure, volume, and temperature for a fixed mass of gas:- P is pressure
- V is volume
- n is the number of moles
- R is the universal gas constant
- T is temperature (K)
- P is the absolute pressure
- V is the volume
- N is the number of gas molecules
- k is the Boltzmann constant (1.381×10−23 J·K−1 in SI units)
- T is the temperature (K)
This law has the following important consequences:
- If temperature and pressure are kept constant, then the volume of the gas is directly proportional to the number of molecules of gas.
- If the temperature and volume remain constant, then the pressure of the gas changes is directly proportional to the number of molecules of gas present.
- If the number of gas molecules and the temperature remain constant, then the pressure is inversely proportional to the volume.
- If the temperature changes and the number of gas molecules are kept constant, then either pressure or volume (or both) will change in direct proportion to the temperature.
Other gas laws
- Graham's law states that the rate at which gas molecules diffuse is inversely proportional to the square root of its density. Combined with Avogadro's law (i.e. since equal volumes have equal number of molecules) this is the same as being inversely proportional to the root of the molecular weight.
- Dalton's law of partial pressures states that the pressure of a mixture of gases simply is the sum of the partial pressures of the individual components. Dalton's Law is as follows:
 ,
,
- Henry's law states that:
- At constant temperature, the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
Graham's laws
- Graham's law, known as Graham's law of effusion, was formulated by Scottish physical chemist Thomas Graham in 1848. Graham found experimentally that the rate of effusion of a gas is inversely proportional to the square root of the mass of its particles. This formula can be written as:

- Rate1 is the rate of effusion of the first gas (volume or number of moles per unit time).
- Rate2 is the rate of effusion for the second gas.
- M1 is the molar mass of gas 1
- M2 is the molar mass of gas 2.
Graham's law is most accurate for molecular effusion which involves the movement of one gas at a time through a hole. It is only approximate for diffusion of one gas in another or in air, as these processes involve the movement of more than one gas.
Example
Let gas 1 be H2 and gas 2 be O2.Graham's Law can also be used to find the approximate molecular weight of a gas if one gas is a known species, and if there is a specific ratio between the rates of two gases (such as in the previous example). The equation can be solved for either one of the molecular weights provided the subscripts are consistent.
Source : http://en.wikipedia.org

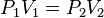






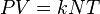

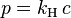




Komentar